COVIRAN vaccine to begin clinical trial on children aged 12-18
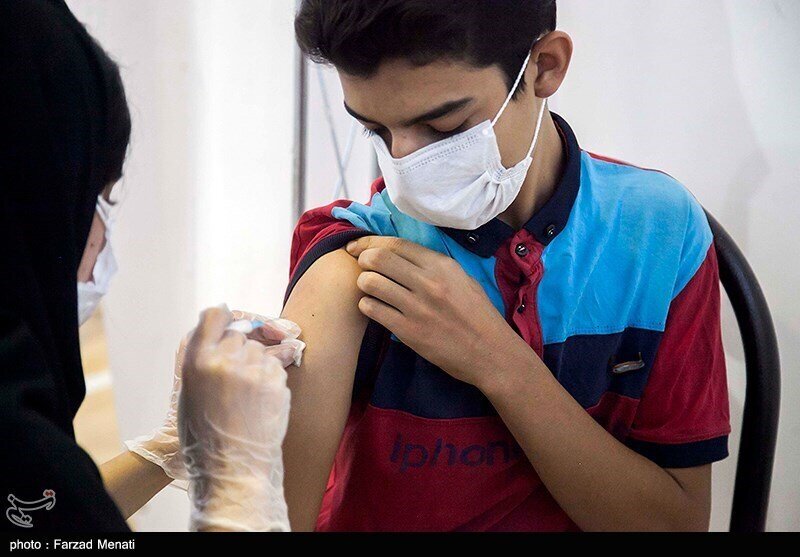
TEHRAN – COVIRAN BAREKAT vaccine received a license to start a clinical trial on children aged 12 to 18 by the next week, Minoo Mohraz, a member of the National Scientific Committee of COVID-19, has said.
Made by researchers at the Headquarters for Executing the Order of the Imam, COVIRAN Barekat was unveiled on December 29, 2020, and received the license for public use on June 14.
Referring to 60 volunteers in the first phase of the clinical trial, she noted that children between the ages of 12 and 18 will enter the study, and if the result is successful, children between the ages of 6 and 12 will also attend the study.
“We will start clinical trials on children based on the existing routine in the world and observing international standards,” she added.
According to the Food and Drug Administration, 14 vaccines are being domestically developed in Iran. Pastu Covac, developed jointly by the Pasteur Institute of Iran and Cuba's Finlay Vaccine Institute, is the only homegrown vaccine that includes use in children aged under 18.
Currently, COVIRAN has produced over 10 million doses of vaccine.
An article on clinical and technical knowledge was published in the Journal of Medical Virology with an impact factor of 98.6 and in the Q1 category.
The vaccine proved effective against Indian strain, according to Hojjat Niki-Maleki, head of the information center of Headquarters for Executing the Order of the Imam.
Eleven countries from Asia and South America, and a European country have asked for importing COVIRAN vaccine, Hassan Jalili, the vaccine’s production manager, said in June.
According to the Food and Drug Administration, 14 vaccines are being domestically developed in the country which are in different study phases.
The second Iranian-made vaccine developed by the Razi Vaccine and Serum Research Institute (Razi Cov Pars) started the clinical trial on February 27.
Fakhra vaccine, the third domestically-developed COVID-19 vaccine, was unveiled and started the clinical trial on March 16.
Lately, the Food and Drug Administration issued an emergency use license for two other domestic vaccines of Razi Cov Pars and Fakhra.
Iran is one of the few countries that has all vaccine production platforms, Mohammad Reza Shanehsaz, former head of the Iranian Food and Drug Administration, said in June.
Meanwhile, World Health Organization (WHO) representative to Iran Jaffar Hussain said in September that the Organization was collecting the necessary information for the registration and certification of Iranian-made coronavirus vaccines.
FB/MG
